Understanding the Deactivation of Fe Single-Atom on Nitrogen-Doped Carbon Catalysts in Oxygen Reduction Reaction.
Mar 20, 2024· ·
0 min read
·
0 min read
Maria Minotaki, PhD
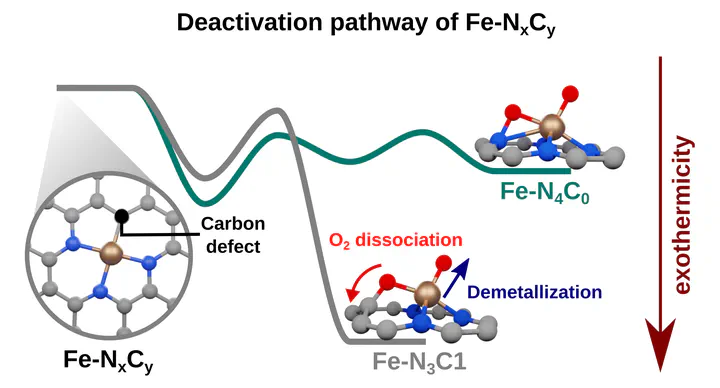 Image credit: American Chemical Society
Image credit: American Chemical SocietyAbstract
Single-atom catalysts (SACs) have gained attention as potential replacements for the platinum-group metals (PGMs) in the oxygen reduction reaction (ORR), a fundamental reaction in renewable energy technologies. Among SACs, the Fe single-atom nitrogen-doped carbon catalysts (Fe–NxCy) are prominent due to their remarkable activity. However, their long-term stability under operando conditions remains a critical challenge. Here, we show that a carbon atom coordinated to the Fe center can activate and interact with reactive oxygen species, leading to the formation of a stabilized C–O bond. Demetallization is promoted by electronic and structural changes in the catalyst, driven by its spin-polarization state. These findings provide mechanistic insights into the deactivation of Fe–NxCy moieties and guide the design of robust and sustainable catalysts for energy applications.